SOMEONE, PLEASE HELP.
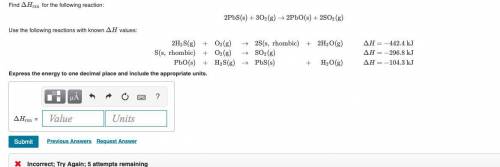
Find ΔHrxn
2PbS(s)+3O2(g)→2PbO(s)+2SO2(g)
Use the follo...

Chemistry, 11.06.2020 02:57 angelafisher886
SOMEONE, PLEASE HELP.
Find ΔHrxn
2PbS(s)+3O2(g)→2PbO(s)+2SO2(g)
Use the following reactions with known ΔH Values:

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:20
One or more substances changing into one or more substances is an example of a
Answers: 1

Chemistry, 22.06.2019 04:20
Which of the following is true for the actual yield of a reaction? it is always calculated as a ratio. it is the yield from the excess reactant. it is the yield from the limiting reactant. it is always less than the theoretical yield.
Answers: 1


You know the right answer?
Questions























