

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Joseph has hypothesized that sound travels in waves. if he were following the scientific method, what should he do next? a. ask a question. b. test the hypothesis. c. study the results. d. tell other scientists about his hypothesis.
Answers: 1

Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1

Chemistry, 23.06.2019 00:30
Gasoline has a density of 0.740 g/ml. if you have 328 grams of gasoline, what is the volume in milliliters?
Answers: 1

Chemistry, 23.06.2019 01:00
Imagine if during the cathode ray experiment, the size of the particles of the ray was the same as the size of the atom forming the cathode. which other model or scientific observation would have also been supported? 1. this would support dalton's postulates that proposed the atoms are indivisible because no small particles are involved. 2. this would support bohr's prediction about electrons moving in orbits having specific energy. 3. this would support bohr's prediction about electrons being randomly scattered around the nucleus in the atom. 4. this would support dalton's postulates that proposed that atoms combine in fixed whole number ratios to form compounds.
Answers: 1
You know the right answer?
According to the ideal gas law, what happens to the volume of a gas when the
temperature doubles (a...
Questions

Mathematics, 09.07.2019 11:00


Biology, 09.07.2019 11:00

English, 09.07.2019 11:00


Mathematics, 09.07.2019 11:00

Mathematics, 09.07.2019 11:00


Chemistry, 09.07.2019 11:00

Biology, 09.07.2019 11:00

Social Studies, 09.07.2019 11:00


Biology, 09.07.2019 11:00





Mathematics, 09.07.2019 11:00

Mathematics, 09.07.2019 11:00

Mathematics, 09.07.2019 11:00

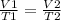 And volume and temperature are directly correlated.
And volume and temperature are directly correlated.

