
Calculate the percentage of the void space out of the total volume occupied by 1 mole of water molecules. The density of water is assumed to be 1.0 g/mL that is 1.0 g/cm3. The molar mass of water is 18.0 g/mol. The atomic radius of hydrogen is 37 pm and of oxygen is 73 pm. The formula for the volume of a sphere is 4/3(r3

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:40
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3

Chemistry, 22.06.2019 10:00
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

Chemistry, 23.06.2019 12:30
You have 125 g of a certain seasoning and are told that it contains 70.0 g of salt. what is the peroentage of salt by mass in this seasoning?
Answers: 2

Chemistry, 23.06.2019 17:00
Achemist tests new mixtures for paint samples and decides one mixture's stickiness makes it worthless. a scientist working in a nearby lab suggests a different use for this sticky mixture. which statement best describes this situation?
Answers: 1
You know the right answer?
Calculate the percentage of the void space out of the total volume occupied by 1 mole of water molec...
Questions







Mathematics, 23.08.2019 22:00


Mathematics, 23.08.2019 22:00

Biology, 23.08.2019 22:00


Biology, 23.08.2019 22:00

Social Studies, 23.08.2019 22:00

Biology, 23.08.2019 22:00

Mathematics, 23.08.2019 22:00

Mathematics, 23.08.2019 22:00

Geography, 23.08.2019 22:00



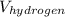 = 2 × 4/3 × π ×
= 2 × 4/3 × π × 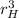
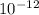 meters
meters

