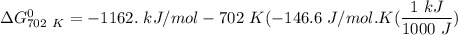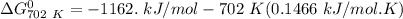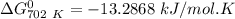Consider the following reaction:
2NO(g)+O2(g)→2NO2(g)
Estimate ΔG∘ for this reaction at each of the following temperatures and predict whether or not the reaction will be spontaneous. (Assume that ΔH∘ and ΔS∘ do not change too much within the give temperature range.) I need to find the temperature are 298K and 702K. For 298K It is simple because at standard temperature
ΔG∘ = DG(products)- DG(reactants).

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Using the periodic table, complete the table to describe each atom. type in your answers.a ? b? c? d? e? f?
Answers: 1

Chemistry, 22.06.2019 02:30
In pea plants, the allele for tallness (t) is dominant to the allele for shortness (t). in the cross between a tall pea plant and a short pea plant shown below, what is the probability that the resulting offspring will be tall? whats the percent
Answers: 1

Chemistry, 22.06.2019 10:00
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1

You know the right answer?
Consider the following reaction:
2NO(g)+O2(g)→2NO2(g)
Estimate ΔG∘ for this reaction at each...
Estimate ΔG∘ for this reaction at each...
Questions


English, 08.12.2020 21:40




Geography, 08.12.2020 21:40

Mathematics, 08.12.2020 21:40


Mathematics, 08.12.2020 21:40



Mathematics, 08.12.2020 21:40

Mathematics, 08.12.2020 21:40


Mathematics, 08.12.2020 21:40

Mathematics, 08.12.2020 21:40




Mathematics, 08.12.2020 21:40

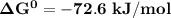 ; as such the reaction is said to be spontaneous since the value of
; as such the reaction is said to be spontaneous since the value of 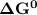 is negative.
is negative.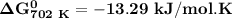 and the reaction is spontaneous
and the reaction is spontaneous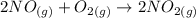
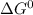 ;
;![\Delta G^0 = [2(\Delta G^0_{NO_{2(g)}}] - [1(\Delta G^0_{O_{2(g)}})+ 2(\Delta G^0_{NO_{g}})]](/tpl/images/0683/6875/905f6.png)
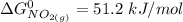
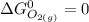
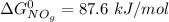
![\Delta G^0 = [2(51.2 \ kJ/mol}] - [1(0)+ 2(87.6 \ kJ/mol})]](/tpl/images/0683/6875/34022.png)
![\Delta G^0 = [102.4 \ kJ/mol}] - [175.2 \ kJ/mol})]](/tpl/images/0683/6875/a6424.png)
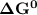 of the reaction when the temperature is 702 K is:
of the reaction when the temperature is 702 K is: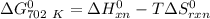
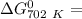 Gibbs free energy of the reaction at 702 K
Gibbs free energy of the reaction at 702 K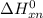 = standard enthalpy of the reaction = -116.2 kJ/mol
= standard enthalpy of the reaction = -116.2 kJ/mol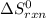 = standard entropy of the reaction = -146.6 J/mol/K
= standard entropy of the reaction = -146.6 J/mol/K