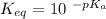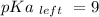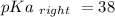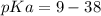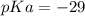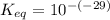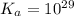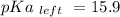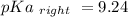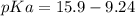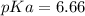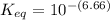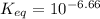Chemistry, 12.06.2020 04:57 katekayrodriguez10
Calculate Keq for these reactions and predict if the equilibrium will lie to the right or to the left as written. (You may enter your answer in scientific notation, e. g. 1.0*10^-9. Enter your answer to two significant figures.) Reaction 1: + + pKa = 9 pKa = 38 Keq = Equilibrium position = Reaction 2: + + pKa = 35 pKa = 25 Keq = Equilibrium position =

Answers: 1
Another question on Chemistry


Chemistry, 21.06.2019 21:30
The reaction q+r2=r2q is found to be first order in r2 and
Answers: 1

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1

Chemistry, 22.06.2019 15:30
The gulf stream is a warm water current that flows away from the equator to northern europe. witch of these does it cause. a. crashes of warm and cool water in the ocean b.colder climates near the equator c.large waves on the cost of europe d.warm climates in northern europe
Answers: 1
You know the right answer?
Calculate Keq for these reactions and predict if the equilibrium will lie to the right or to the lef...
Questions


English, 12.12.2020 16:50

Mathematics, 12.12.2020 16:50

Mathematics, 12.12.2020 16:50

Mathematics, 12.12.2020 16:50

Biology, 12.12.2020 16:50




Mathematics, 12.12.2020 16:50


Spanish, 12.12.2020 16:50

Mathematics, 12.12.2020 16:50

Mathematics, 12.12.2020 16:50

Mathematics, 12.12.2020 16:50




Computers and Technology, 12.12.2020 16:50

Mathematics, 12.12.2020 16:50

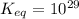
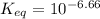
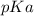 is mathematically evaluated as
is mathematically evaluated as 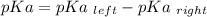
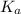 is mathematically evaluated as
is mathematically evaluated as