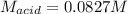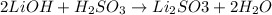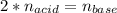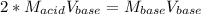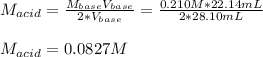Chemistry, 11.06.2020 20:57 DeGeneral770
A chemist uses a standard solution of 0.210 M lithium hydroxide (LiOH) to titrate 28.10 mL of sulfurous acid acid (H2SO3), she finds that it requires 22.14 mL of the base to reach the end-point of the titration. What is the molarity of the acid solution? What is the concentration of H2SO3? Your answer must be rounded to the correct number of significant figures. Be sure to specify a unit.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:30
In an energy pyramid, which level has the most available energy?
Answers: 1

Chemistry, 22.06.2019 01:20
Match the acid base pairs by arranging the acid name with the conjugate base formula. hydrogen carbonate hydrogen phosphate carbonic acid read water sulfuric acid phosphoric acid a. co32- b. hso4- c. hco3- d. po43- e. h2po4- f. oh-
Answers: 1

Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3

You know the right answer?
A chemist uses a standard solution of 0.210 M lithium hydroxide (LiOH) to titrate 28.10 mL of sulfur...
Questions

Mathematics, 04.09.2020 19:01




Spanish, 04.09.2020 19:01

Mathematics, 04.09.2020 19:01




Mathematics, 04.09.2020 19:01




History, 04.09.2020 19:01

English, 04.09.2020 19:01

Mathematics, 04.09.2020 19:01

Geography, 04.09.2020 19:01

History, 04.09.2020 19:01

Mathematics, 04.09.2020 19:01

Business, 04.09.2020 19:01