
Chemistry, 13.06.2020 01:57 lovelybear2354
Given the balanced equation: Pb(NO₃)₂(aq) + 2NaCl(aq) ➔ PbCl₂(s) + 2NaNO₃(aq). What type of reaction is represented by this equation

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:10
How do forces between particles in gases compare to forces in the other states of matter? o a. the forces in gases are stronger than forces in solids but weaker than forces in liquids. o b. the forces in gases are weaker than forces in solids but stronger than forces in liquids. o c. the forces in gases are weaker than forces in solids and liquids. o d. the forces in gases are stronger than forces in solids and liquids. submit
Answers: 1

Chemistry, 22.06.2019 01:10
Which of the following elements would you expect to have the lowest ionization energy value? fluorine, lithium, neon, nitrogen
Answers: 2

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 18:50
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1
You know the right answer?
Given the balanced equation: Pb(NO₃)₂(aq) + 2NaCl(aq) ➔ PbCl₂(s) + 2NaNO₃(aq). What type of reaction...
Questions



Social Studies, 16.10.2019 21:40

Mathematics, 16.10.2019 21:40

English, 16.10.2019 21:40


Mathematics, 16.10.2019 21:40

Social Studies, 16.10.2019 21:40

History, 16.10.2019 21:40


Mathematics, 16.10.2019 21:40



Geography, 16.10.2019 21:40

Spanish, 16.10.2019 21:40


Chemistry, 16.10.2019 21:40

Mathematics, 16.10.2019 21:40

Mathematics, 16.10.2019 21:40

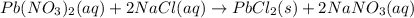
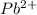 and
and 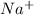 ions switched places. Also, both
ions switched places. Also, both 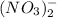 and
and 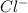 ions switched places.
ions switched places.

