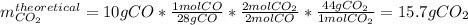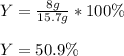Chemistry, 13.06.2020 05:57 michaelwarren8728
Consider the reaction 2 CO + O2 → 2 CO2 .What is the percent yield of carbon dioxide
(MW = 44 g/mol) if the reaction of 10 g of carbon monoxide (MW = 28 g/mol) with
excess O2 produces 8 g of carbon dioxide? Answer in units of %.

Answers: 2
Another question on Chemistry


Chemistry, 23.06.2019 03:00
Achemical equilibrium between gaseous reactants and products is shown. n2(g) + 3h2(g) ⇌ 2nh3(g) how will the reaction be affected if the pressure on the system is increased? it will shift toward the reactant side as there is lower pressure on the reactant side. it will shift toward the product side as there is higher pressure on the product side. it will shift toward the reactant side as there are a greater number of moles of gas on the reactant side. it will shift toward the product side as there are a fewer number of moles of gas on the product side.
Answers: 2

Chemistry, 23.06.2019 05:00
Match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) amount of product predicted to be produced by the given reactants theoretical yield c) reactant that can produce more of the product
Answers: 3

Chemistry, 23.06.2019 05:30
What is the morality of 2.50 l of solution that contains 1.85 mol of anhydrous sodium tetraborate?
Answers: 1
You know the right answer?
Consider the reaction 2 CO + O2 → 2 CO2 .What is the percent yield of carbon dioxide
(MW = 44 g/mol...
Questions

History, 25.06.2019 05:30


Mathematics, 25.06.2019 05:30

Mathematics, 25.06.2019 05:30

Mathematics, 25.06.2019 05:30

Mathematics, 25.06.2019 05:30

Mathematics, 25.06.2019 05:30

Mathematics, 25.06.2019 05:30


Social Studies, 25.06.2019 05:30





Mathematics, 25.06.2019 05:40


Social Studies, 25.06.2019 05:40



Mathematics, 25.06.2019 05:40