I NEED HELP PLEASE, THANKS!
Ammonia (NH3) is an example of a Brønsted-Lowry Base.
-Define the...

Chemistry, 13.06.2020 09:57 hmskevinjacobo5471
I NEED HELP PLEASE, THANKS!
Ammonia (NH3) is an example of a Brønsted-Lowry Base.
-Define the Brønsted-Lowry acid-base theory.
-What is the pH of an ammonia solution that has a concentration of 0.335 M? The Kb of ammonia is 1.8 × 10^–5.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:30
For the following dehydrohalogenation (e2) reaction, draw the zaitsev product(s) resulting from elimination involving c3–c4 (i.e., the carbon atoms depicted with stereobonds). show the product stereochemistry clearly. if there is more than one organic product, both products may be drawn in the same box. ignore elimination involving c3 or c4 and any carbon atom other than c4 or c3.
Answers: 3

Chemistry, 22.06.2019 09:10
Select the correct answer from each drop-down menu.describe what happens to a carbon-11 atom when it undergoes positron emission.the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3

Chemistry, 22.06.2019 11:30
If we compare and contrast electromagnetic waves with sound waves, all but one statement is true. that is a) sound waves require a medium to travel while electromagnetic waves do not. b) electromagnetic waves can travel through the vacuum of space while sound waves cannot. c) electromagnetic waves must have a medium in which to travel, but sound waves can travel anywhere. eliminate d) sound waves must bounce off of matter in order to travel while electromagnetic waves do not require matter to be present.
Answers: 3

Chemistry, 23.06.2019 10:00
How many moles are equal to 2.4×10^23 formula units of sodium chloride
Answers: 1
You know the right answer?
Questions

History, 17.09.2021 19:00

Health, 17.09.2021 19:00


Physics, 17.09.2021 19:00




Chemistry, 17.09.2021 19:00

Business, 17.09.2021 19:00




English, 17.09.2021 19:00


Biology, 17.09.2021 19:00


History, 17.09.2021 19:00


Computers and Technology, 17.09.2021 19:00

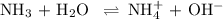
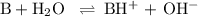
![\rm K_{\text{b}} = \dfrac{\text{[BH}^{+}]\text{[OH}^{-}]}{\text{[B]}} = 1.8 \times 10^{-5}\\\\\dfrac{x^{2}}{0.335 - x} = 1.8 \times 10^{-5}](/tpl/images/0685/0322/975fe.png)
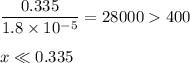
![\dfrac{x^{2}}{0.335} = 1.8 \times 10^{-5}\\\\x^{2} = 0.335 \times 1.8 \times 10^{-5}\\x^{2} = 6.03 \times 10^{-6}\\x = \sqrt{6.03 \times 10^{-6}}\\x = \text{[OH]}^{-} = \mathbf{2.46 \times 10^{-3}} \textbf{ mol/L}](/tpl/images/0685/0322/0aa42.png)
![\text{pOH} = -\log \text{[OH}^{-}] = -\log(2.46 \times 10^{-3}) = 2.61](/tpl/images/0685/0322/acbc3.png)


