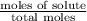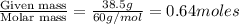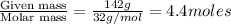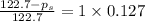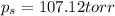Chemistry, 13.06.2020 23:57 reginaldlegette
Calculate the vapor pressure (in torr) at 298 K in a solution prepared by dissolving 38.5 g of the non-volatile non-electrolye urea {CO(NH2)2} in 142 g of methanol. The vapor pressure of methanol at 298 K is 122.7 torr. Give your answer to 2 decimal places.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:40
What is the total reduction potential of a cell in which potassium (k) is reduced and copper (cu) is oxidized? a. 2.59 v b. 3.27 v c. -3.27 v d.-2.59 v
Answers: 1

Chemistry, 22.06.2019 02:00
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1


Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3
You know the right answer?
Calculate the vapor pressure (in torr) at 298 K in a solution prepared by dissolving 38.5 g of the n...
Questions



Mathematics, 03.01.2020 20:31

Mathematics, 03.01.2020 20:31





History, 03.01.2020 20:31





History, 03.01.2020 20:31

Geography, 03.01.2020 20:31

History, 03.01.2020 20:31

Mathematics, 03.01.2020 20:31

Mathematics, 03.01.2020 20:31


English, 03.01.2020 20:31


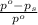 = relative lowering in vapor pressure
= relative lowering in vapor pressure = mole fraction of solute =
= mole fraction of solute =