Hydrogen bromide decomposes when heated to 437C according to the equation: 2HBr(g) H2(g) Br2(g). If the reaction starts with 2.15 mol of hydrogen bromide in 1.0 liter, and decomposes to 36.7%, what is the equilibrium constant of the decomposition of hydrogen bromide

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:00
The diagram shows the positions of the sun, moon and earth during spring tides, when the high tides are at their highest and low tides at their lowest. what is it about these positions that causes these high and low tides?
Answers: 3

Chemistry, 22.06.2019 09:00
Chemical energy is a form of a. kinetic energy only. b. both potential and kinetic energy. c. neither potential nor kinetic energy. d. potential energy only. reset
Answers: 1

Chemistry, 22.06.2019 12:40
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3
You know the right answer?
Hydrogen bromide decomposes when heated to 437C according to the equation: 2HBr(g) H2(g) Br2(g). If...
Questions



Mathematics, 08.02.2022 07:50


English, 08.02.2022 07:50

Physics, 08.02.2022 07:50

Mathematics, 08.02.2022 07:50

Mathematics, 08.02.2022 07:50

Mathematics, 08.02.2022 07:50

Mathematics, 08.02.2022 07:50








Mathematics, 08.02.2022 07:50


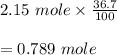
![K_c=\frac{[H_2][Br_2]}{[HBr]^2} \\\\=\frac{(0.395)(0.395)}{(1.361)^2} \\\\=\frac{0.156025}{1.852321} \\\\=0.084](/tpl/images/0685/5664/e5d29.png)