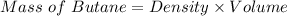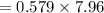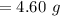Be sure to answer all parts. Butane gas is compressed and used as a liquid fuel in disposable cigarette lighters and lightweight camping stoves. Suppose a lighter contains 7.96 mL of butane (d = 0.579 g/mL). (a) How many grams of oxygen are needed to burn the butane completely?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:00
Achemist wants to extract copper metal from copper chloride solution. the chemist places 0.50 grams of aluminum foil in a solution containing 0.75 grams of copper (ii) chloride. a single replacement reaction takes place. (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction? a) approximately 0.36 grams, because copper (ii) chloride acts as a limiting reactant b) approximately 1.8 grams, because copper (ii) chloride acts as a limiting reactant c) approximately 0.36 grams, because aluminum acts as a limiting reactant d) approximately 1.8 grams, because aluminum acts as a limiting reactant
Answers: 3

Chemistry, 22.06.2019 20:30
How many grams of phosphorus are contained in 5.09 moles of phosphorus?
Answers: 1

Chemistry, 23.06.2019 01:30
Concentrations expressed as a percent by mass are useful when the solute is a a. liquid b. gas c. solid
Answers: 1

Chemistry, 23.06.2019 08:00
Pl what kind of reaction is this? nahco3 + h2o → co2 + naoh + h2o -composition -decomposition -single replacement -double replacement im leaning more toward single replacement. if im wrong can you explain whyy?
Answers: 2
You know the right answer?
Be sure to answer all parts. Butane gas is compressed and used as a liquid fuel in disposable cigare...
Questions

History, 08.02.2021 19:50

Spanish, 08.02.2021 19:50



Mathematics, 08.02.2021 19:50



Social Studies, 08.02.2021 19:50


Computers and Technology, 08.02.2021 19:50




Mathematics, 08.02.2021 19:50

Mathematics, 08.02.2021 19:50

History, 08.02.2021 19:50



Mathematics, 08.02.2021 19:50

Mathematics, 08.02.2021 19:50