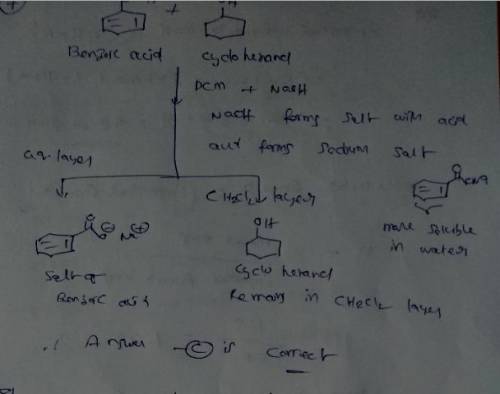
What would happen if a mixture of benzoic acid and cyclohexanol dissolved in CH2Cl2 is treated with aqueous NaOH solution? Group of answer choices The salt of benzoic acid would dissolve in the aqueous layer while cyclohexanol would remain in the CH2Cl2 layer. Benzoic acid would remain in the CH2Cl2 layer, and cyclohexanol would dissolve in the aqueous layer. Benzoic acid would dissolve in the aqueous layer while cyclohexanol would remain in the CH2Cl2 layer. The salt of benzoic acid would remain in the CH2Cl2 layer while cyclohexanol would dissolve in the aqueous layer.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:30
Asa choose the correct set of reaction coefficients to properly balance the following chemical equation according to the law of conservation of mass: __s8 + __o2 ==> __so2 1, 1, 8 1, 8, 1 1, 8, 8 8, 1, 1
Answers: 1

Chemistry, 22.06.2019 18:00
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1


You know the right answer?
What would happen if a mixture of benzoic acid and cyclohexanol dissolved in CH2Cl2 is treated with...
Questions


Computers and Technology, 01.07.2019 19:00

Mathematics, 01.07.2019 19:00

History, 01.07.2019 19:00





Mathematics, 01.07.2019 19:00



Biology, 01.07.2019 19:00

History, 01.07.2019 19:00

History, 01.07.2019 19:00


Mathematics, 01.07.2019 19:00

Geography, 01.07.2019 19:00


Mathematics, 01.07.2019 19:00

Mathematics, 01.07.2019 19:00