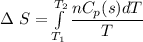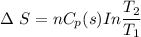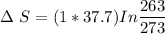Chemistry, 14.06.2020 10:57 nate102201
1 mol super cooled liquid water transformed to solid ice at -10 oC under 1 atm pressure.
a) Calculate entropy change of the system, surrounding and universe. (temperature of the
environment is -10 °C)
b) Make some comments on entropy changes from the obtained data.
Please use the following data for water :
Melting entalpy of ice (ΔHmelting) at 0°C and 1 bar is 6020 J mol-1
.
Cp (H2O (s)) = 37,7 J mol-1 K-1
Cp (H2O (l)) = 75,3 J mol-1 K-1

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 13:00
Identify the balanced chemical equation that represents a decomposition reaction. p4 + 3o2 ⟶ p2o3 2fe(oh)3 ⟶ 2feo3 + h2o cuso4 ⟶ cuo + 2so3 2fe(oh)3 ⟶ fe2o3 + 3h2o
Answers: 1

Chemistry, 22.06.2019 05:00
Which position represents spring in the southern hemisphere? a) b) c) d)
Answers: 2

Chemistry, 22.06.2019 20:30
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1

Chemistry, 23.06.2019 03:00
Which of the following is a chemical property of water at 4 c
Answers: 2
You know the right answer?
1 mol super cooled liquid water transformed to solid ice at -10 oC under 1 atm pressure.
a) Calcula...
Questions


History, 21.10.2020 21:01




Advanced Placement (AP), 21.10.2020 21:01



History, 21.10.2020 21:01


Mathematics, 21.10.2020 21:01



Mathematics, 21.10.2020 21:01



History, 21.10.2020 21:01

Mathematics, 21.10.2020 21:01

Mathematics, 21.10.2020 21:01

Biology, 21.10.2020 21:01