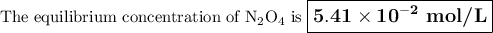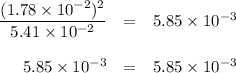Consider the following equilibrium:
N204(9)
2NO2(9)
Ket
= 5.85 x 10-3
Whi...

Chemistry, 16.06.2020 01:57 Aliciaonfleek
Consider the following equilibrium:
N204(9)
2NO2(9)
Ket
= 5.85 x 10-3
Which statement about this system is true?
Options:
The equilibrium lies to the left.
The equilibrium lies to the right.
If the equilibrium concentration of No, is 1.78 x 10-2 m, the equilibrium concentration of
N204 is
Options:
3.04 M.
1.85 × 10^-6 M.
5.42 × 10^-2 M.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:30
Reaction rate depends on how many molecules are coming into contact with each other with enough energy to react. increasing the temperature of the reactants will increase -
Answers: 3

Chemistry, 22.06.2019 17:00
The biosphere of the earth is made up of what compound? organic or inorganic?
Answers: 3

Chemistry, 22.06.2019 19:30
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1

Chemistry, 22.06.2019 21:00
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1
You know the right answer?
Questions

Mathematics, 26.08.2019 13:20

History, 26.08.2019 13:20



Mathematics, 26.08.2019 13:20


Social Studies, 26.08.2019 13:30

Spanish, 26.08.2019 13:30



Social Studies, 26.08.2019 13:30


English, 26.08.2019 13:30


Mathematics, 26.08.2019 13:30

Mathematics, 26.08.2019 13:30

Mathematics, 26.08.2019 13:30

Mathematics, 26.08.2019 13:30


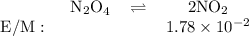
![K_{\text{c}} = \dfrac{\text{[NO$_{2}$]}^{2}}{\text{[N$_{2}$O$_{4}$]}} = \dfrac{(1.78 \times 10^{-2})^{2}}{\text{[N$_{2}$O$_{4}$]}} = 5.85 \times 10^{-3}\\\\\begin{array}{rcl}\\(1.78 \times 10^{-2})^{2}&=&\text{[N$_{2}$O$_{4}$]} \times 5.85 \times 10^{-3}\\\text{[N$_{2}$O$_4$]}&=& \dfrac{(1.78 \times 10^{-2})^{2}}{5.85 \times 10^{-3}}\\\\& = & \mathbf{5.41 \times 10^{-2}}\textbf{ mol/L}\\\end{array}\\](/tpl/images/0686/6303/751d3.png)