

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:20
Calculate the molarity of the solution. 6.02 x 1022 molecules of hci (molecular weight = 36.5 g/mole) in 2.0 liters of water m
Answers: 1

Chemistry, 22.06.2019 13:30
Which of the following has wavelength longer than the wavelength of viable light? a) x rays b) gamma rays c) radios waves d) ultraviolet waves
Answers: 1

Chemistry, 22.06.2019 23:10
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 3

Chemistry, 23.06.2019 02:00
Why does ammonia, nh3, behave as a base when it reacts with an acid? z
Answers: 2
You know the right answer?
What volume of 0.110 M Hydrochloric acid needs to be added to 30 mL of 0.100 M ammonia to make a buf...
Questions


Mathematics, 25.05.2020 14:57

Physics, 25.05.2020 14:57


History, 25.05.2020 14:57

Mathematics, 25.05.2020 14:57

Mathematics, 25.05.2020 14:57

Mathematics, 25.05.2020 14:57

Mathematics, 25.05.2020 14:57

History, 25.05.2020 14:57

English, 25.05.2020 14:57

Social Studies, 25.05.2020 14:57

Mathematics, 25.05.2020 14:57

Biology, 25.05.2020 14:57

Arts, 25.05.2020 14:57

English, 25.05.2020 14:57


Mathematics, 25.05.2020 14:57

Mathematics, 25.05.2020 14:57

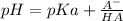 (Equation 1)
(Equation 1)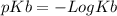 (equation 2) and
(equation 2) and 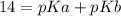 (equation 3) With this in mind we can do the calculations:
(equation 3) With this in mind we can do the calculations: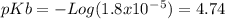
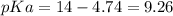
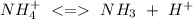 (reaction 1)
(reaction 1)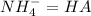 and
and 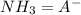 . Now if we use the hendersson-hasselbach reaction we can find the ratio (
. Now if we use the hendersson-hasselbach reaction we can find the ratio (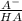 ) that we need for the desired pH value, so:
) that we need for the desired pH value, so:
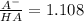 (equation 4)
(equation 4) ), so:
), so: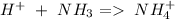 (reaction 2)
(reaction 2)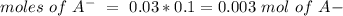
 before the addition:
before the addition: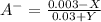 (equation 5)
(equation 5) (equation 6)
(equation 6)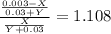 (equation 7)
(equation 7)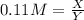 (equation 8)
(equation 8)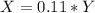 (equation 9)
(equation 9)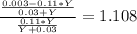 (equation 10).
(equation 10).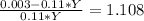 (equation 11)
(equation 11)

