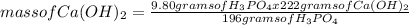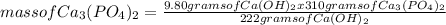Chemistry, 16.06.2020 07:57 perezsamantha3oqr0za
What is the maximum amount of Ca3(PO4)2 that can be prepared from 9.80 g of Ca(OH)2 and 9.80 g of
H3PO4
Ca(OH)2 (s) + H3PO4 (aq)
Ca3(PO4)2 (aq) + H2O (1)
balance the equation 1st.
O 6.80 g
O 15.5 g
O 8.60 g
o 13.7 g
O 10.3 g

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:00
Look at the bean data from days 4–6. use these data to explain how natural selection changed the number of dark red walking beans over time. writing part
Answers: 3

Chemistry, 22.06.2019 05:30
What royal scientist used the 29th day of frozen vapor to encounter elements for mastering new culinary creations?
Answers: 1


Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1
You know the right answer?
What is the maximum amount of Ca3(PO4)2 that can be prepared from 9.80 g of Ca(OH)2 and 9.80 g of
H...
Questions









Mathematics, 27.06.2019 04:30




English, 27.06.2019 04:30





Social Studies, 27.06.2019 04:30

Health, 27.06.2019 04:30

Biology, 27.06.2019 04:30