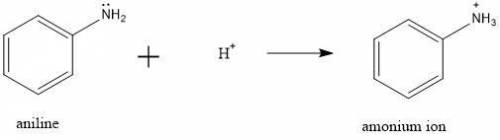Chemistry, 16.06.2020 20:57 rayniqueamee2002
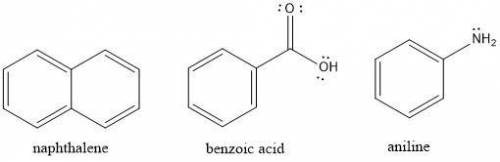
Suppose you have crude reaction mixture containing napthalene, benzoic acid, and aniline dissolved in an organic solvent, and you wish to extract the different molecules by altering the solubility of each component in solution. Which of the following statements would be true?
a. Adding 5% HCl solution to the crude reaction mixture will deprotonate benzoic acid increasing its solubility in the aqueous solution.
b. Adding 5% NaOH solution to the crude reaction mixture will protonate napthalene making it more soluble in the aqueous solution.
c. Adding 5% HCl solution to the crude reaction mixture will protonate napthalene making it more soluble in the aqueous solution.
d. Adding 5% HCl solution to the crude reaction mixture will protonate aniline increasing its solubility in the aqueous solution.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which of the following pairs of elements belong to the same groupa. h and he b. li and bec. c and pb d. ga and ge
Answers: 1

Chemistry, 22.06.2019 14:30
Which of the following is not one of the steps in the scientific method a. hypothesize b. summarize c. analyze d. familiarize
Answers: 3

Chemistry, 22.06.2019 17:30
What type of organic molecule comprises the majority of a potato?
Answers: 1

Chemistry, 22.06.2019 18:00
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3
You know the right answer?
Suppose you have crude reaction mixture containing napthalene, benzoic acid, and aniline dissolved i...
Questions

Social Studies, 26.10.2021 20:40


Mathematics, 26.10.2021 20:40

Social Studies, 26.10.2021 20:40

Mathematics, 26.10.2021 20:40

Mathematics, 26.10.2021 20:40

English, 26.10.2021 20:40

Mathematics, 26.10.2021 20:40

Mathematics, 26.10.2021 20:40

Mathematics, 26.10.2021 20:40


Mathematics, 26.10.2021 20:40

Social Studies, 26.10.2021 20:40






English, 26.10.2021 20:40

Mathematics, 26.10.2021 20:40

 the presence of this hydronium ion will protonate the acid, so we can discard a.
the presence of this hydronium ion will protonate the acid, so we can discard a.