
Recall that atoms gain or lose electrons to become lons. So, an lon has a different number of electrons than its
corresponding neutral atom. Positive ions have lost electrons, and negative lons have gained electrons.
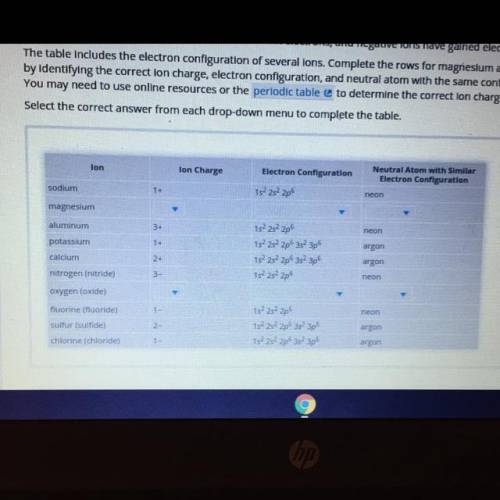
The table includes the electron configuration of several lons. Complete the rows for magnesium and oxygen (oxide) lons
by identifying the correction charge, electron configuration, and neutral atom with the same configuration as the lon.
You may need to use online resources or the periodic table to determine the correct lon charges.
Select the correct answer from each drop-down menu to complete the table.
lon
Ion Charge
Electron Configuration
Neutral Atom with similar
Electron Configuration
neon
sodium
1+
15²25²205
magnesium
3+
neon
aluminum
potassium
calcium
1+
152 2s 2p
152 2s 2p 3s 3p
1s 2s 2p 3s 3p
152 2s 2p
argon
argon
2-
nitrogen (nitride)
3-
neon
oxygen (oxide)
neon
fluorine (fluoride)
sulfur (sulfide)
chlorine (chloride)
2-
152 2s 2p
15²252 226 35² 3p6
152 2s 2p 3s 3p
argon
argon
1-

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:00
The graph above shows how the price of cell phones varies with the demand quantity. the equilibrium price for cell phones is where both supply and demand quantities equal $100, 5,000 5,000, $100
Answers: 2

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 06:00
Oxidation-reduction reactions (often called "redox" for short) are reactions that involve the transfer of electrons from one species to another. oxidation states, or oxidation numbers, allow chemists to keep track of these electron transfers. in general, one element will lose electrons (oxidation), with the result that it will increase in oxidation number, and another element will gain electrons (reduction), thereby decreasing in oxidation number. the species that is oxidized is called the reducing agent or reductant. the species that is reduced is called the oxidizing agent or oxidant. to sum up: oxidation = increase in oxidation state = loss of electrons = reducing agent reduction = decrease in oxidation state = gain of electrons = oxidizing agent part a which element is oxidized in this reaction? fe2o3+3co→2fe+3co2 enter the elemental symbol. view available hint(s) is oxidized part b which element is reduced in this reaction? 2hcl+2kmno4+3h2c2o4→6co2+2mno2+2kcl+4h2o enter the elemental symbol. view available hint(s) is reduced
Answers: 1

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2
You know the right answer?
Recall that atoms gain or lose electrons to become lons. So, an lon has a different number of electr...
Questions



Mathematics, 14.01.2021 01:00




History, 14.01.2021 01:00

Mathematics, 14.01.2021 01:00

Mathematics, 14.01.2021 01:00

Mathematics, 14.01.2021 01:00

English, 14.01.2021 01:00


History, 14.01.2021 01:00

Mathematics, 14.01.2021 01:00


Medicine, 14.01.2021 01:00

Mathematics, 14.01.2021 01:00



Physics, 14.01.2021 01:00



