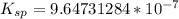Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 09:10
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Chemistry, 22.06.2019 10:30
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2

Chemistry, 22.06.2019 14:30
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1
You know the right answer?
Using the solubility of Ca(IO3)2 that you determined in 0.01M KIO3 as a solvent, calculate the value...
Questions

Mathematics, 22.01.2021 23:20

Chemistry, 22.01.2021 23:20


English, 22.01.2021 23:20


Mathematics, 22.01.2021 23:20

Geography, 22.01.2021 23:20

Mathematics, 22.01.2021 23:20

History, 22.01.2021 23:20


Mathematics, 22.01.2021 23:20

Mathematics, 22.01.2021 23:20

Computers and Technology, 22.01.2021 23:20

Mathematics, 22.01.2021 23:20




Health, 22.01.2021 23:20


Mathematics, 22.01.2021 23:20

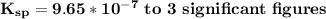
 can be calculated by the formula:
can be calculated by the formula:![K_{sp} = [Ca^{2+}][IO^-_3]^2](/tpl/images/0687/2933/f1f21.png)
![K_{sp} = [0.00341][0.01682]^2](/tpl/images/0687/2933/ef259.png)
![K_{sp} = [0.00341][2.829124*10^{-4}]](/tpl/images/0687/2933/d4986.png)