
Chemistry, 17.06.2020 10:57 yeahmaneee
Calculate [NO3-] if 125 mL of 0.35 M NaNO3 is mixed with 450 mL of 1.1 M Mg(NO3)2. Please include some of your work as best as you can in the answer for full marks.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 19:30
List the two type of transporst that the cell in orde to transport molecules acroos the membrane
Answers: 1

Chemistry, 21.06.2019 21:00
Match the following items. 1. high-intensity bundle of energy being emitted from some decaying nuclei gamma ray 2. particle radiating from the nucleus of some atoms beta particle 3. negative particle identical to an electron but radiating from a decaying nucleus alpha particle
Answers: 1

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1

Chemistry, 22.06.2019 07:30
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1
You know the right answer?
Calculate [NO3-] if 125 mL of 0.35 M NaNO3 is mixed with 450 mL of 1.1 M Mg(NO3)2. Please include so...
Questions

Mathematics, 07.11.2020 06:20

Mathematics, 07.11.2020 06:20

Mathematics, 07.11.2020 06:20






History, 07.11.2020 06:20


Mathematics, 07.11.2020 06:20

English, 07.11.2020 06:20

Physics, 07.11.2020 06:20

Mathematics, 07.11.2020 06:20



French, 07.11.2020 06:20


Mathematics, 07.11.2020 06:20

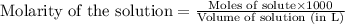 .....(1)
.....(1)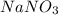 solution = 0.35 M
solution = 0.35 M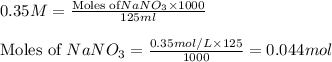
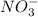
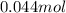 of
of 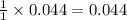 mol of
mol of 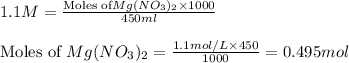
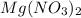 contains = 2 mol of
contains = 2 mol of 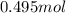 of
of 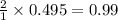 mol of
mol of ![[NO_3^-]=\frac {\text {total moles}}{\text {total volume}}=\frac{0.044+0.99}{0.575L}=1.76M](/tpl/images/0687/7799/56220.png)
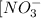 after mixing is 1.76 M
after mixing is 1.76 M

