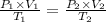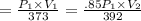Chemistry, 17.06.2020 19:57 deshawnnash53
For many purposes we can treat methane (CH) as an ideal gas at temperatures above its boiling point of - 161. °C. Suppose the temperature of a sample of methane gas is raised from 100.0 °C to 119.0 °C, and at the same time the pressure is decreased by 15.0%. increase Does the volume of the sample increase, decrease, or stay the same? decrease x 6 ? stays the same If you said the volume increases or decreases, calculate the percentage change in the volume. Round your answer to the nearest percent. 1%

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Use the drop-down menus to answer each question. which runner finished the 100 m race in the least amount of time? which runner stopped running for a few seconds during the race? at what distance did anastasia overtake chloe in the race?
Answers: 1

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3


Chemistry, 22.06.2019 22:00
What mass of glucose is produced when 54g of water react with carbon dioxide
Answers: 1
You know the right answer?
For many purposes we can treat methane (CH) as an ideal gas at temperatures above its boiling point...
Questions

History, 19.08.2019 01:30

History, 19.08.2019 01:30

Mathematics, 19.08.2019 01:30

Biology, 19.08.2019 01:30

Mathematics, 19.08.2019 01:30

English, 19.08.2019 01:30





Mathematics, 19.08.2019 01:30



English, 19.08.2019 01:30

Social Studies, 19.08.2019 01:30



Mathematics, 19.08.2019 01:30

Mathematics, 19.08.2019 01:30

Mathematics, 19.08.2019 01:30