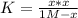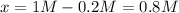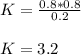Chemistry, 18.06.2020 05:57 andregijoe41
PCl5 dissociates according to the reaction: PCl5(g) = PCl3(g) + Cl2(g) One mole of PCl5 was placed in one liter of solution. When equilibrium was established, 0.2 mole of PCl5 remained in the mixture. What is the equilibrium constant for this reaction? (Hint: remember the ICE procedure? initial, change, and equilibrium)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 10:30
Aglow stick contains a glass vial with chemicals. when the glow stick is bent, the vial breaks and the chemicals react to produce a glow. a science student observes that a glow stick kept in the freezer glows for a longer duration than a glow stick kept at room temperature. what conclusion can be drawn based on the observation? be sure to note the outcome and test variables in the conclusion.
Answers: 1

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3

Chemistry, 22.06.2019 17:10
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following? a) the need for a coenzymeb) allosteric inhibitionc) competitive inhibitiond) insufficient cofactors
Answers: 1
You know the right answer?
PCl5 dissociates according to the reaction: PCl5(g) = PCl3(g) + Cl2(g) One mole of PCl5 was placed i...
Questions



History, 18.10.2019 19:30


Mathematics, 18.10.2019 19:30

Law, 18.10.2019 19:30


Health, 18.10.2019 19:30

Geography, 18.10.2019 19:30

Biology, 18.10.2019 19:30



Mathematics, 18.10.2019 19:30

Mathematics, 18.10.2019 19:30

History, 18.10.2019 19:30


Health, 18.10.2019 19:30


Mathematics, 18.10.2019 19:30

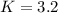
![K=\frac{[PCl_3][Cl_2]}{[PCl_5]}](/tpl/images/0688/6864/bc353.png)
 due to the reaction extent (ICE procedure):
due to the reaction extent (ICE procedure):