
Chemistry, 18.06.2020 07:57 joslynndiggs
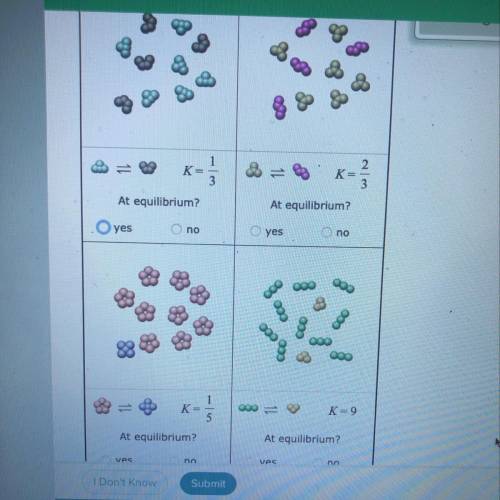
Tiny samples of aqueous solutions are sketched below, as if under a microscope so powerful that individual molecules could be seen. (The water molecules are
not shown.)
The two substances in each sample can interconvert. That is, each kind of molecule can turn into the other. The equilibrium constant K for each interconversion
equilibrium is shown below the sketch.
Decide whether each solution is at equilibrium.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:00
How many moles of magnesium is 3.01 x10^22 atoms of magnesium?
Answers: 1

Chemistry, 22.06.2019 05:40
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1

Chemistry, 22.06.2019 09:00
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

Chemistry, 22.06.2019 10:10
What shape would a molecule with two bound groups and two lone pairs have?
Answers: 1
You know the right answer?
Tiny samples of aqueous solutions are sketched below, as if under a microscope so powerful that indi...
Questions









Biology, 26.02.2020 19:31


Chemistry, 26.02.2020 19:31



Mathematics, 26.02.2020 19:31








