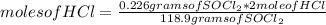Chemistry, 18.06.2020 19:57 QuestionsAnsweredNow
How many moles of HCl can be produced from 0.226 g of SOCl2? SOCl2 + H2O > SO2 + 2HCl Explanation would be helpful c:

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:40
How many liters of hydrogen gas will be produced at stp from the reaction of 7.179×10^23 atoms of magnesium with 54.219g of phosphoric acid (h3po4) the equation is 3mg + 2h3(> mg(po4)2+3h2
Answers: 1

Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2

Chemistry, 22.06.2019 20:30
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1

Chemistry, 22.06.2019 22:00
The volume of an unknown substance in a sealed glass jar is 50 milliliters. the volume of the jar is 200 milliliters. which state of matter could the substance be?
Answers: 2
You know the right answer?
How many moles of HCl can be produced from 0.226 g of SOCl2? SOCl2 + H2O > SO2 + 2HCl
Explanatio...
Questions

Mathematics, 06.06.2020 03:59



Geography, 06.06.2020 03:59


Mathematics, 06.06.2020 03:59

Mathematics, 06.06.2020 03:59

English, 06.06.2020 03:59

Mathematics, 06.06.2020 03:59


Mathematics, 06.06.2020 03:59

Mathematics, 06.06.2020 03:59

Mathematics, 06.06.2020 03:59


History, 06.06.2020 03:59




Chemistry, 06.06.2020 03:59