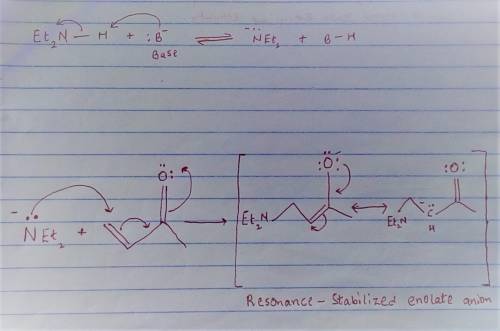
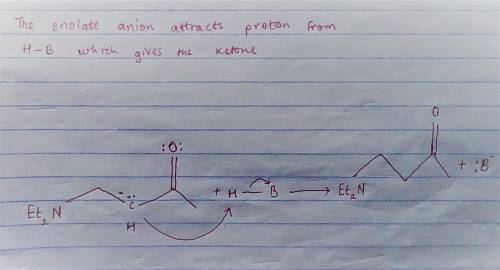
Chemistry, 19.06.2020 00:57 josephvcarter
Carbon-carbon double bonds are electron-rich regions and are attacked by electrophiles (for example, ); they are not attacked by nucleophiles (for example, diethylamine, ). + reaction arrow with electrophilic addition written above + no reaction However, when the carbon-carbon double bond has a carbonyl group adjacent to it, the double bond reacts readily with nucleophiles by nucleophilic addition. + reaction arrow with nucleophilic addition written above For the following reaction, draw the structure of the resonance contributor that is attacked by diethylamine. + Include all valence lone pairs in your answer.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Choose all the answers that apply. ionic compounds dissolve easily in water do not dissolve in water have low melting points have high melting points conduct electricity when melted
Answers: 1

Chemistry, 22.06.2019 09:30
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3


Chemistry, 23.06.2019 05:30
Elizabeth has two separate samples of the same substance. sample is in the liquid state, and the other is in the solid state. the two samples most likely differ in which property?
Answers: 1
You know the right answer?
Carbon-carbon double bonds are electron-rich regions and are attacked by electrophiles (for example,...
Questions

Mathematics, 22.07.2021 07:10

Mathematics, 22.07.2021 07:10



History, 22.07.2021 07:10


Mathematics, 22.07.2021 07:10


Computers and Technology, 22.07.2021 07:10

Chemistry, 22.07.2021 07:10



English, 22.07.2021 07:10


Engineering, 22.07.2021 07:20


Mathematics, 22.07.2021 07:20

Mathematics, 22.07.2021 07:20

Mathematics, 22.07.2021 07:20

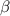 - carbon of the conjugate thereby resulting into a resonance stabilized enolate anion.
- carbon of the conjugate thereby resulting into a resonance stabilized enolate anion.