Chemistry, 19.06.2020 03:57 extasisjorge
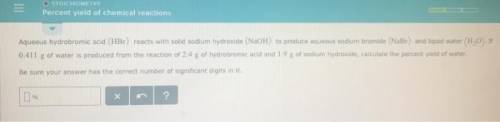
Aqueous hydrobromic acid reacts with solid sodium hydroxide to produce aqueous sodium bromide and liquid water . If of water is produced from the reaction of of hydrobromic acid and of sodium hydroxide, calculate the percent yield of water. Round your answer to significant figures.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:00
The image shows a process that releases nuclear energy which statement best identifies the process shown the process must be fusion because energy is released the process must be fusion because of have your nucleus formed a smaller nuclei the process must be fission because a large nucleus breaks into smaller nuclei the process must be fission because neutrons are formed
Answers: 1

Chemistry, 22.06.2019 04:20
Which of the following is true for the actual yield of a reaction? it is always calculated as a ratio. it is the yield from the excess reactant. it is the yield from the limiting reactant. it is always less than the theoretical yield.
Answers: 1

Chemistry, 22.06.2019 07:00
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1

Chemistry, 22.06.2019 07:10
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2
You know the right answer?
Aqueous hydrobromic acid reacts with solid sodium hydroxide to produce aqueous sodium bromide and li...
Questions

Mathematics, 30.01.2020 18:54

Mathematics, 30.01.2020 18:54

English, 30.01.2020 18:54

Mathematics, 30.01.2020 18:54


English, 30.01.2020 18:54

Mathematics, 30.01.2020 18:54

History, 30.01.2020 18:54





Spanish, 30.01.2020 18:55


Mathematics, 30.01.2020 18:55


Social Studies, 30.01.2020 18:55



Mathematics, 30.01.2020 18:55