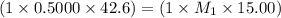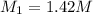Chemistry, 18.06.2020 15:57 umezinwachukwuebuka1
Ammonia (NH3) is a weak base that reacts with a strong acid to form the ammonium ion, NH4 . In the titration of 15.00 mL of an ammonia cleaner with 0.5000 M HCl, 42.6 mL of the titrant was required to reach the endpoint. What is the concentration of the NH3 in solution

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:50
H2so4(aq) + mg(s)—> mgso4(aq) +h2(g) which substance is the acid in the reaction?
Answers: 3

Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 21:30
Athe top of a hill, an athlete on a skateboard has x joules of mechanical energy. how much mechanical energy will she have at the bottom of the hill? ignore the effects of friction.
Answers: 1

Chemistry, 23.06.2019 04:20
Malia was able to make a paper clip float on the surface of water what will most likely happen to the paper clip if a drop of dishwashing detergent is added near it
Answers: 1
You know the right answer?
Ammonia (NH3) is a weak base that reacts with a strong acid to form the ammonium ion, NH4 . In the t...
Questions

Mathematics, 24.03.2021 19:40


Physics, 24.03.2021 19:40




Mathematics, 24.03.2021 19:40

Mathematics, 24.03.2021 19:40


Mathematics, 24.03.2021 19:40

Mathematics, 24.03.2021 19:40


Mathematics, 24.03.2021 19:40

French, 24.03.2021 19:40

English, 24.03.2021 19:40



Mathematics, 24.03.2021 19:40

Advanced Placement (AP), 24.03.2021 19:40

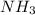 in solution is 1.42 M
in solution is 1.42 M
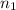 = basicity of
= basicity of 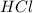 = 1
= 1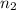 = acidity of
= acidity of 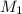 = concentration of
= concentration of 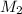 = concentration of
= concentration of 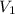 = volume of
= volume of 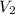 = volume of
= volume of