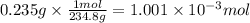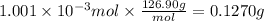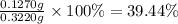Chemistry, 19.06.2020 03:57 smartboy2296
A sample of 0.3220 g of an ionic compound containing the iodide ion (I-) is dissolved in water and treated with an excess of AgHCO3. If the mass of the AgI precipitate that forms is 0.235 g, what is the percent by mass of I in the original compound? The molar mass of AgI is 234.8 g.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:40
Consider the nuclear equation below. 239/94 pu—-> x+ 4/2 he. what is x?
Answers: 2

Chemistry, 22.06.2019 06:00
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2

Chemistry, 22.06.2019 07:30
Aradio signal from a gps satellite take only about 0.067 seconds to reach a gps reciever. if the speed of light is about 300,000km/s, then approximately how far away is the reciever from from the satellite?
Answers: 1

You know the right answer?
A sample of 0.3220 g of an ionic compound containing the iodide ion (I-) is dissolved in water and t...
Questions


Mathematics, 06.04.2021 01:00

History, 06.04.2021 01:00


Mathematics, 06.04.2021 01:00





History, 06.04.2021 01:00

Computers and Technology, 06.04.2021 01:00




Mathematics, 06.04.2021 01:00





Mathematics, 06.04.2021 01:00