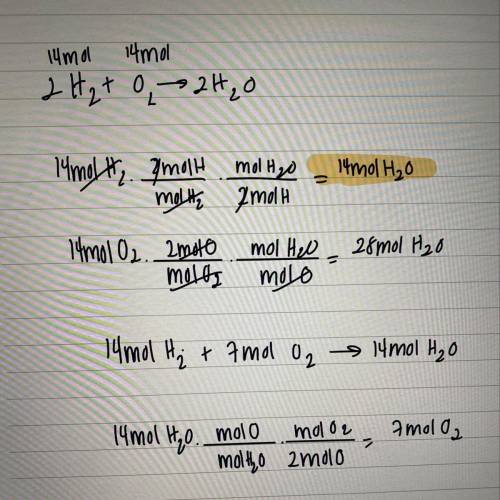Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Acurium-245 nucleus is hit with a neutron and changes as shown by the equation. complete the equation by filling in the missing parts. 52
Answers: 2

Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2

Chemistry, 22.06.2019 08:40
Which statement can best be concluded from the ideal gas law?
Answers: 2

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2
You know the right answer?
If 14 moles of oxygen react with 14 moles of hydrogen to produce water, what is the
limiting reacta...
Questions






English, 18.11.2019 20:31

Mathematics, 18.11.2019 20:31

Mathematics, 18.11.2019 20:31

English, 18.11.2019 20:31



Biology, 18.11.2019 20:31

History, 18.11.2019 20:31



Social Studies, 18.11.2019 20:31