

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:00
During chemistry class, carl performed several lab tests on two white solids. the results of three tests are seen in the data table. based on this data, carl has concluded that substance b must have bonds.
Answers: 2

Chemistry, 22.06.2019 08:00
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1

Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2

Chemistry, 22.06.2019 20:00
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1
You know the right answer?
Determine the chemical amount of chlorine required to produce 2.50 mol of lithium chloride, LiCl, ac...
Questions

Computers and Technology, 24.06.2019 23:00




Mathematics, 24.06.2019 23:00

History, 24.06.2019 23:00


Mathematics, 24.06.2019 23:00


Mathematics, 24.06.2019 23:00

History, 24.06.2019 23:00




Biology, 24.06.2019 23:00




Mathematics, 24.06.2019 23:00

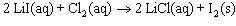
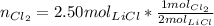 Units for molLiCl cancel out
Units for molLiCl cancel out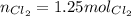 <= notice that we keep 3 significant figures
<= notice that we keep 3 significant figures

