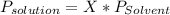Chemistry, 20.06.2020 05:57 kodiebclay
The vapor pressure of diethyl ether (ether) is 463.6 mm Hg at 25 °C. How many grams of aspirin , C9H8O4, a nonvolatile, nonelectrolyte (MW = 180.1 g/mol), must be added to 219.0 grams of diethyl ether to reduce the vapor pressure to 458.5 mm Hg ? diethyl ether = CH3CH2OCH2CH3 = 74.12 g/mol.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 13:30
1) which of the following is the best example of a physical change? a) sugar dissolving in tea b) firefly glowing 2) in the combustion of ethane, what is/are the reactants? c2h6 + o2 ==> co2 + h2o a) c2h6 and o2 b) co2 and c2h6
Answers: 2

Chemistry, 22.06.2019 20:00
Acm ruler with main graduations from 1 to 10 from left to right there are 10 secondary graduations between each of the main graduations there is a line that begins. at the left end of the ruler 10 secondary graduations to the left of the “1 main graduation the right end of the line ends on the eighth secondary graduation to the right of 3 how long is the line
Answers: 1


Chemistry, 23.06.2019 08:30
If you had to research a particular disease or area of concern in veterinary medicine and science, which one would you choose? why?
Answers: 1
You know the right answer?
The vapor pressure of diethyl ether (ether) is 463.6 mm Hg at 25 °C. How many grams of aspirin , C9H...
Questions


Mathematics, 11.03.2021 01:40


English, 11.03.2021 01:40




Chemistry, 11.03.2021 01:40







Mathematics, 11.03.2021 01:40

Biology, 11.03.2021 01:40