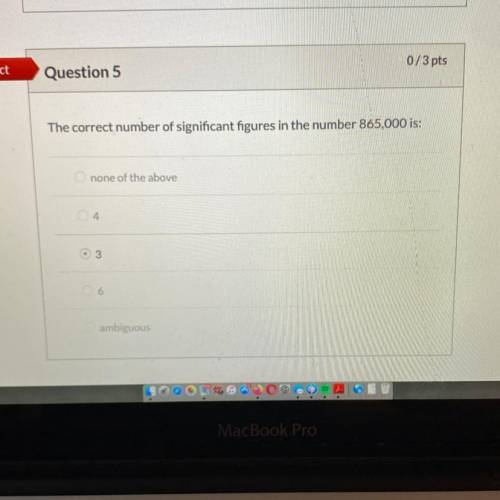
Using the rules of significant figures, calculate the following: 1.2315+
0.116 + 15.10 is.
16...

Chemistry, 19.06.2020 07:57 lightbright828
Using the rules of significant figures, calculate the following: 1.2315+
0.116 + 15.10 is.
16.45
• 16.4
16
16.4475
0/3 nts

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:30
The atomic radius of sodium is 186 pm and of chlorine is 100 pm. the ionic radius for na+ is 102 pm and for cl– is 181 pm. in going from na to cl in period 3, why does the atomic radius decrease while the ionic radius increases? a. the inner electrons in the sodium cation shield its valence electrons more effectively than the inner electrons in the chloride anion do. b. the inner electrons shield the valence electrons more effectively in the chlorine atom than in the chloride anion. c. the outermost electrons in chloride experience a smaller effective nuclear charge than those in the sodium cation do. d. the outermost electrons in chloride experience a larger effective nuclear charge than those in the sodium cation do. e. monatomic ions are bigger than the atoms from which they are formed.
Answers: 2

Chemistry, 22.06.2019 09:10
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Chemistry, 22.06.2019 21:30
While in europe, if you drive 125 km per day, how much money would you spend on gas in one week if gas costs 1.10 euros per liter and your car’s gas mileage is 32.0 mi/gal? assume that 1 euro=1.26 dollars
Answers: 2

You know the right answer?
Questions

Mathematics, 17.09.2019 18:30



Biology, 17.09.2019 18:30

History, 17.09.2019 18:30

Mathematics, 17.09.2019 18:30



Physics, 17.09.2019 18:30

History, 17.09.2019 18:30

Mathematics, 17.09.2019 18:30

Chemistry, 17.09.2019 18:30

History, 17.09.2019 18:30

Mathematics, 17.09.2019 18:30



Mathematics, 17.09.2019 18:30



English, 17.09.2019 18:30



