
Chemistry, 20.06.2020 08:57 graciewyatt471
1) How many kJ are absorbed when 45.2 g of water at 31.3 oC is heated to 76.9 oC? 2) Calculate the total heat released in kcal when 72.1 g water at 25.2 oC is cooled to 0 oC and freezes. 3) How many kilojoules are required to heat 55,500 mg of gold with specific heat = 0.129 J/g oC is heated from 24.6 oC to 123.4 oC? 4) Calculate the heat needed in kcal to change 45.6 g of water at 100 oC to change into steam.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:30
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 12:00
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1

Chemistry, 22.06.2019 14:30
An object resting on a table weighs 100 n. with what force is the object pushing on the table? with what force is the table pushing on the object? explain how you got your answer.
Answers: 3

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 1
You know the right answer?
1) How many kJ are absorbed when 45.2 g of water at 31.3 oC is heated to 76.9 oC? 2) Calculate the t...
Questions


Mathematics, 18.09.2021 01:00


Computers and Technology, 18.09.2021 01:00


Computers and Technology, 18.09.2021 01:00

Mathematics, 18.09.2021 01:00

History, 18.09.2021 01:00





Mathematics, 18.09.2021 01:00




Health, 18.09.2021 01:00



Mathematics, 18.09.2021 01:00

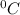 and Δθ is the change in temperature.
and Δθ is the change in temperature.

