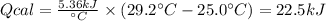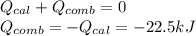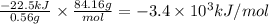Chemistry, 20.06.2020 12:57 stormserena
A 0.56 g sample of liquid C6H12 was combusted completely using excess oxygen inside a bomb (constant volume) calorimeter, with the products being carbon dioxide and liquid water. The calorimeter's heat capacity is 5.36 kJ °C-1. If the temperature inside the calorimeter increased from 25.0 °C to 29.2 °C, determine ΔrH for this reaction in kJ mol-1 (with respect to C6H12) at 298 K. Do not worry about how realistic the final answer is. You have 5 attempts at this question. TIP: To report an answer in scientific notation, enter it using the format "2.3E4", which means "2.3 x 104" (without the quotation marks)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 21:20
Juju.01) 5 geologic events a group of students designed an experiment in an ice rink to represent the solar system. the steps of the experiment are listed below. 5: geologic events 1. choose a student to represent the sun. the planets are represented by two tennis balls. 2. ask the student to hold the tennis balls in each palm and spin on the ice with arms stretched out. 3. ask the student to draw in the arms after about 10 spins. 4. observe the student's arms rotate faster when they are closer to the body. 05 geologic events enors) the experiment most likely demonstrates that (2 points) 07 discussion-based sessment/module planets exert gravitational force on the sun the sun exerts gravitational force on the planets 3.07 discussion-based ssessment speed of a planet depends on its distance from the sun new version available! (3.0.119) get it now submit 18.07: module exam description
Answers: 3

Chemistry, 23.06.2019 07:40
)in the deacon process for the manufacture of chlorine, hcl and o2 react to form cl2 and h2o. sufficient air (21 mole% o2, 79% n2) is fed to provide 35% excess oxygen, and the fractional conversion of hcl is 85%. calculate the mole fractions of the product stream components.
Answers: 1


Chemistry, 23.06.2019 14:30
Will give imagine you are given a mystery element. it is, however, a discovered and known element. you may perform a maximum of two observations or tests to determine its identity. time and money is critical, so you need to prioritize your tests. if you can get by with a single test, you get 100 super-geek points from your research lab team. pick your two tests, number them as #1 and #2, and justify why you think these two will certainly be enough (and why the first might well be enough all by itself.) the available tests are classification into metal, non-metal, or metalloid, count of valence electrons, count of electron shells, atomic radius (error range: +/- 1 pm), electronegativity (error range: +/- 0.1), first ionization energy (error range: +/- 10 kj/mole), melting point (error range: +/- 10 c), and boiling point (error range: +/- 20 c).
Answers: 2
You know the right answer?
A 0.56 g sample of liquid C6H12 was combusted completely using excess oxygen inside a bomb (constant...
Questions

Social Studies, 15.01.2021 02:20

Mathematics, 15.01.2021 02:20


Geography, 15.01.2021 02:20



History, 15.01.2021 02:20

Mathematics, 15.01.2021 02:20


History, 15.01.2021 02:20

Spanish, 15.01.2021 02:20

Mathematics, 15.01.2021 02:20


Physics, 15.01.2021 02:20

Mathematics, 15.01.2021 02:20



Mathematics, 15.01.2021 02:20

Mathematics, 15.01.2021 02:20

Mathematics, 15.01.2021 02:20

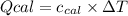
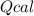 : heat absorbed by the calorimeter
: heat absorbed by the calorimeter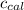 : heat capacity of the calorimeter
: heat capacity of the calorimeter : change in the temperature
: change in the temperature