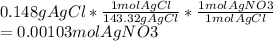Chemistry, 19.06.2020 17:57 kittenface3428
To lift fingerprints from a crime scene, a solution of silver nitrate is sprayed on a surface to react with the sodium chloride left behind by perspiration. What is the molarity of a silver nitrate solution if 42.8 mL of it reacts with excess sodium chloride to produce 0.148g of precipitate according to the following reaction?
AgNO3(aq)+NaCl(aq) --> AgCl(s)+NaNO3(aq)
A) 2.41 x 10^-2 M
B) 0.0229 M
C) 6.66 x 10^-2 M
D) 3.2 x 10^-3 M
E) 2.29 x 10^2 M

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 10:10
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate,m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3

Chemistry, 23.06.2019 00:00
How many atoms or molecules are there in a mole of a substance?
Answers: 1

Chemistry, 23.06.2019 00:30
Five different substances are given to you to be dissolved in water. which substances are most likely to undergo dissolution in water? check all that apply. view available hint(s) check all that apply. sodium fluoride, naf octane, c8h18 propanol, ch3ch2ch2oh potassium iodide, ki benzene, c6h6
Answers: 1
You know the right answer?
To lift fingerprints from a crime scene, a solution of silver nitrate is sprayed on a surface to rea...
Questions



Business, 04.01.2020 01:31


Social Studies, 04.01.2020 01:31

Social Studies, 04.01.2020 01:31

Business, 04.01.2020 01:31



Social Studies, 04.01.2020 01:31









English, 04.01.2020 01:31