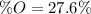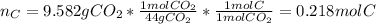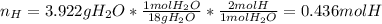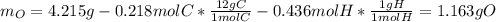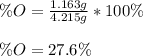Chemistry, 20.06.2020 17:57 collin0123
A 4.215 g sample of a compound containing only carbon, hydrogen, and oxygen is burned in an excess of oxygen gas, producing 9.582 g CO2 and 3.922 g H2O. What percent by mass of oxygen is contained in the original sample?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Cucl2 + 2nano3 cu(no3)2 + 2nacl what is the percent yield of nacl if 31.0 g of cucl2 reacts with excess nano3 to produce 21.2 g of nacl? 49.7% 58.4% 63.6% 78.7%
Answers: 1

Chemistry, 22.06.2019 08:30
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3

Chemistry, 22.06.2019 09:00
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1
You know the right answer?
A 4.215 g sample of a compound containing only carbon, hydrogen, and oxygen is burned in an excess o...
Questions

Physics, 19.08.2021 01:40


Mathematics, 19.08.2021 01:40



Mathematics, 19.08.2021 01:40

Mathematics, 19.08.2021 01:40

Mathematics, 19.08.2021 01:40


Mathematics, 19.08.2021 01:40


Business, 19.08.2021 01:40

Mathematics, 19.08.2021 01:40






Mathematics, 19.08.2021 01:40

Mathematics, 19.08.2021 01:50