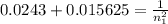Chemistry, 20.06.2020 18:57 tatianaware1617
The electron in a hydrogen atom, originally in level n = 8, undergoes a transition to a lower level by emitting a photon of wavelength 3745 nm. What is the final level of the electron? (c = 3.00 x 108m/s, h = 6.63 x 10−34 J • s, RH = 2.179 x 10−18 J)
a. 5
b. 6
c. 8
d. 9
e. 1

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 3

Chemistry, 22.06.2019 22:30
Molecular iodine, i2(g), dissociates into iodine atoms at 625 k with a first-order rate constant of 0.271 s−1. part a part complete what is the half-life for this reaction?
Answers: 3

Chemistry, 23.06.2019 08:30
This has nothing to do with school. i wrote a poem to my crush, who i'm asking out soon. tell me if it's cheesy, or cute. "roses are red, violets are blue no love story sounds right if it doesn't include you. dance with me all night, gaze into my eyes i'll hand you my heart, as well as my pride. when i hear your name, my heart goes insane. your all that i want, all that i need promise me you'll stay with me. here it is the final line, jasmine hill will you be mine? " i'm also going to buy her flowers, teddy bear and some food lol. written by me, bre (:
Answers: 2

Chemistry, 23.06.2019 09:30
The mass of a proton is approximately equal to the mass of
Answers: 1
You know the right answer?
The electron in a hydrogen atom, originally in level n = 8, undergoes a transition to a lower level...
Questions




Law, 11.02.2021 01:50

Mathematics, 11.02.2021 01:50

Spanish, 11.02.2021 01:50

Computers and Technology, 11.02.2021 01:50



History, 11.02.2021 01:50



History, 11.02.2021 01:50

Chemistry, 11.02.2021 01:50

English, 11.02.2021 01:50

English, 11.02.2021 01:50



Mathematics, 11.02.2021 01:50

Mathematics, 11.02.2021 01:50

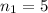
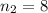
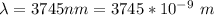

![k = R_{\infty} [\frac{1}{n_1^2} + \frac{1}{n_2^2} ]](/tpl/images/0690/9055/ac88e.png)
 is the Rydberg constant, with a value
is the Rydberg constant, with a value 
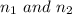 are the principal quantum levels
are the principal quantum levels ![0.0243= [\frac{1}{n_1^2} - \frac{1}{8^2} ]](/tpl/images/0690/9055/cbffd.png)