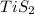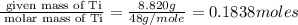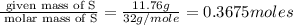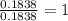Chemistry, 20.06.2020 23:57 saraaaaaaaa20
A student wants to determine the empirical formula for titanium sulfide (TixSy). To do so, she reacted titanium with excess sulfur in a crucible, and recorded the following data: Mass of Crucible: 11.120 g Mass of titanium used: 8.820 g Mass of Crucible and product: 31.700 g what is the empirical formula of titanium sulfide based on her experiment?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Describe the interaction that occurs between two objects with the same electrical charge.
Answers: 1

Chemistry, 22.06.2019 23:00
What does a numerical subscript following an element in a chemical formula mean?
Answers: 1

Chemistry, 23.06.2019 00:00
#7 how does the structure of amino acids allow them to form a polypeptide? each amino acid has an amino group and a carboxyl group. each amino acid has a hydrogen atom and a carboxyl group. each amino acid has a carboxyl group and an r group. each amino acid has an r group and a hydrogen atom.
Answers: 1

Chemistry, 23.06.2019 00:20
Steam reforming of methane ( ch4) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. an industrial chemist studying this reaction fills a 1.5 l flask with 3.5 atm of methane gas and 1.3 atm of water vapor at 43.0°c. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of carbon monoxide gas to be 1 .0 atm. calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 1
You know the right answer?
A student wants to determine the empirical formula for titanium sulfide (TixSy). To do so, she react...
Questions







English, 17.11.2019 15:31






Mathematics, 17.11.2019 15:31


Mathematics, 17.11.2019 15:31


English, 17.11.2019 15:31



Mathematics, 17.11.2019 15:31