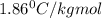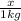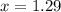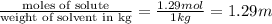Chemistry, 22.06.2020 00:57 Tianylee2328
How many moles of ethylene glycol must be added to 1 kg of water to make a solution with a freezing point of -2.4°C? The freezing point depression constant for water is 1.86°C•kg/mol. What is the molality of the solution?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 15:30
Which of the following mining methods disrupts the sea floor?
Answers: 1

Chemistry, 21.06.2019 21:00
Of the groups of elements below, which are most likely to gain electrons to become anions? a. alkali metal b. boron group c. halogen d. transition metal
Answers: 2

Chemistry, 22.06.2019 07:00
The variability in marine salinity between habitats does not impact the fish living there. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1
You know the right answer?
How many moles of ethylene glycol must be added to 1 kg of water to make a solution with a freezing...
Questions


Mathematics, 17.10.2020 03:01


Mathematics, 17.10.2020 03:01

Mathematics, 17.10.2020 03:01






Chemistry, 17.10.2020 03:01

History, 17.10.2020 03:01


Mathematics, 17.10.2020 03:01

Mathematics, 17.10.2020 03:01

Mathematics, 17.10.2020 03:01




Mathematics, 17.10.2020 03:01

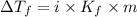
 = Depression in freezing point
= Depression in freezing point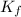 = freezing point constant for water=
= freezing point constant for water=