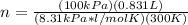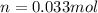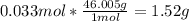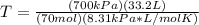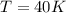PLEASE HELP CHEM BABES I HAVE BEEN CRYING FOR A WHILE NOW
1. Calculate the mass of nitrogen dioxide (NO2) present in a 0.831 L container if the pressure is 100 kPa at a temperature of 27 oC. R = 8.31 kPa x L / mol x K. (K = oC + 273).
2. A 33.2 L tank contains 280 g of compressed helium. If the pressure inside the tank is 700.0 kPa, what is the temperature of the compressed gas? You must convert the mass of helium into moles using the molar mass of He. The conversion factor will be 1 mol / molar mass of helium. R = 8.31 kPa x L / mol x K

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

Chemistry, 22.06.2019 18:30
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1

You know the right answer?
PLEASE HELP CHEM BABES I HAVE BEEN CRYING FOR A WHILE NOW
1. Calculate the mass of nitrogen dioxide...
Questions






Mathematics, 27.01.2021 17:40

Social Studies, 27.01.2021 17:40


History, 27.01.2021 17:40


English, 27.01.2021 17:40

Mathematics, 27.01.2021 17:40



Physics, 27.01.2021 17:40


Mathematics, 27.01.2021 17:40


Mathematics, 27.01.2021 17:40

Mathematics, 27.01.2021 17:40