Chemistry, 23.06.2020 22:01 haylee1468
For the iodine trichloride molecule: a. Determine the number of valence electrons for each atom in the molecule b. Draw the Lewis Dot structure c. Describe why the molecule is drawn this way (i. e. any extra rules/steps needed?) d. Show the polarity of each bond and for the molecule by drawing in the dipole +à

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2

Chemistry, 22.06.2019 19:00
Which statement best describes what happens when molecular compounds melt
Answers: 1

Chemistry, 22.06.2019 20:30
How many grams of phosphorus are contained in 5.09 moles of phosphorus?
Answers: 1
You know the right answer?
For the iodine trichloride molecule: a. Determine the number of valence electrons for each atom in t...
Questions

French, 16.05.2021 08:10


Mathematics, 16.05.2021 08:10

Mathematics, 16.05.2021 08:10




Mathematics, 16.05.2021 08:10


Mathematics, 16.05.2021 08:10

Mathematics, 16.05.2021 08:10



Mathematics, 16.05.2021 08:10


Mathematics, 16.05.2021 08:10



Mathematics, 16.05.2021 08:10

Mathematics, 16.05.2021 08:10

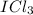 , so the central atom would be "I" and the "Cl" atoms would be placed around "I". See figure 1
, so the central atom would be "I" and the "Cl" atoms would be placed around "I". See figure 1