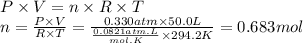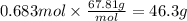Boron trifluoride gas is collected at in an evacuated flask with a measured volume of . When all the gas has been collected, the pressure in the flask is measured to be . Calculate the mass and number of moles of boron trifluoride gas that were collected. Round your answer to significant digits.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 12:30
Which of the following can be used to measure electricity
Answers: 1

Chemistry, 22.06.2019 15:20
Which description best characterizes the motion of particles in a solid?
Answers: 2

Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1

Chemistry, 22.06.2019 23:00
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1
You know the right answer?
Boron trifluoride gas is collected at in an evacuated flask with a measured volume of . When all the...
Questions

Biology, 29.09.2019 06:30

Mathematics, 29.09.2019 06:30

Mathematics, 29.09.2019 06:30

History, 29.09.2019 06:30



Mathematics, 29.09.2019 06:30

Health, 29.09.2019 06:30




Biology, 29.09.2019 06:30



English, 29.09.2019 06:30




History, 29.09.2019 06:30

Biology, 29.09.2019 06:30