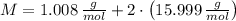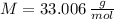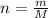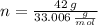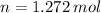Chemistry, 23.06.2020 16:01 leahstubbs
How many moles of \ce{H2O}HX 2 O will be produced from 42.0 \text{ g}42.0 g42, point, 0, start text, space, g, end text of \ce{H2O2}HX 2 OX 2 ?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:30
Asa choose the correct set of reaction coefficients to properly balance the following chemical equation according to the law of conservation of mass: __s8 + __o2 ==> __so2 1, 1, 8 1, 8, 1 1, 8, 8 8, 1, 1
Answers: 1

Chemistry, 22.06.2019 11:10
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1

Chemistry, 22.06.2019 22:30
Which of the following is true about the speed of light? it depends on the wavelength.
Answers: 3

Chemistry, 23.06.2019 01:20
Use the de broglie's wave equation to find the wavelength of an electron moving at 7.3 × 106 m/s. show your work. note: h = plank's constant (6.62607 x 10-34 j s)
Answers: 1
You know the right answer?
How many moles of \ce{H2O}HX 2 O will be produced from 42.0 \text{ g}42.0 g42, point, 0, start text...
Questions



Social Studies, 07.07.2019 23:30


Mathematics, 07.07.2019 23:30

English, 07.07.2019 23:30


Biology, 07.07.2019 23:30

Biology, 07.07.2019 23:30

Biology, 07.07.2019 23:30

Mathematics, 07.07.2019 23:30




Computers and Technology, 07.07.2019 23:30


English, 07.07.2019 23:30

Biology, 07.07.2019 23:30


History, 07.07.2019 23:30

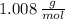 and
and 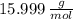 , respectively. A mole is the ratio of current mass of water to molecular weight of water, the latter one is now calculated before computing the amount of moles of water:
, respectively. A mole is the ratio of current mass of water to molecular weight of water, the latter one is now calculated before computing the amount of moles of water: