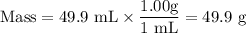Chemistry, 24.06.2020 06:01 maevemboucher78
8. A 50.0 mL 0.05 mol/l solution of sodium cloride (NaCl) was mixed with 100.0 mL
of 0.02 mol/l NaCl solution. What is the mass percent of NaCl in the final solution?
Assume the volumes are additive and their densities 21 g/mL. The molar mass of
NaCl is 58.5 g/mol. (10 points)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:00
An example of technology is the a. addition of a side group to an organic molecule during synthesis. b. use of a new antibiotic to fight an infection. c. measurement of iron concentration in a water sample. d. study of atomic fusion reactions
Answers: 3

Chemistry, 21.06.2019 22:20
Much of the general structure and physical properties of the interior of the earth are inferred from: a)deep oil and gas bore holes b)geologic investigations c)analysis of seismic waves d) study of volcanoes
Answers: 1

Chemistry, 22.06.2019 01:30
There are main groups in the modern periodic table of elements
Answers: 1

Chemistry, 22.06.2019 06:30
What is the correct term for living the most sustainable life you can within your current circumstances?
Answers: 1
You know the right answer?
8. A 50.0 mL 0.05 mol/l solution of sodium cloride (NaCl) was mixed with 100.0 mL
of 0.02 mol/l NaC...
Questions




Mathematics, 11.10.2019 16:30


Mathematics, 11.10.2019 16:30

Mathematics, 11.10.2019 16:30

Mathematics, 11.10.2019 16:30


Biology, 11.10.2019 16:30


Health, 11.10.2019 16:30




Mathematics, 11.10.2019 16:30

English, 11.10.2019 16:30


Mathematics, 11.10.2019 16:30