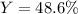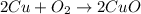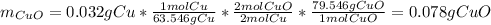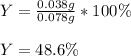Chemistry, 24.06.2020 19:01 jennamcasey94
4. A student started with a 0.032 g sample of copper which he took through the series of reactions described in this experiment. At the end of the experiment he obtained 0.038 g of a black product. What was his percent yield

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1

Chemistry, 22.06.2019 19:00
Structure of the atoms: discovery of the nucleus in 1909i need answering all of these questions
Answers: 3

Chemistry, 22.06.2019 20:20
Which symbol can be used to indicate the pressure at which a chemical reaction is carried out? 25°c 2 atm pa
Answers: 2

You know the right answer?
4. A student started with a 0.032 g sample of copper which he took through the series of reactions d...
Questions

History, 12.05.2021 15:50

Mathematics, 12.05.2021 15:50

German, 12.05.2021 15:50



Mathematics, 12.05.2021 16:00



Geography, 12.05.2021 16:00


Mathematics, 12.05.2021 16:00


Mathematics, 12.05.2021 16:00

Mathematics, 12.05.2021 16:00

Mathematics, 12.05.2021 16:00


Mathematics, 12.05.2021 16:00

Biology, 12.05.2021 16:00