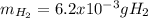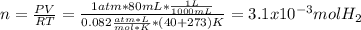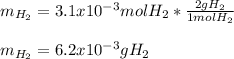Chemistry, 24.06.2020 22:01 keirarae2005
Sometimes in lab we collect the gas formed by a chemical reaction over water. This makes it easy to isolate and measure the amount of gas produced. Suppose H2 the gas evolved by a certain chemical reaction taking place at 40°C is collected over water, using an apparatus something like that in the sketch, and the final volume of gas in the collection tube is measured to be 80ml .
Required:
Calculate the mass of H2 that is in the collection tube. Round your answer to significant digits. You can make any normal and reasonable assumption about the reaction conditions and the nature of the gases.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Explain why scientists use shared characteristics to make cladograms.
Answers: 1

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

Chemistry, 22.06.2019 21:00
One similarity and one difference between an element and a mixture of elements
Answers: 1
You know the right answer?
Sometimes in lab we collect the gas formed by a chemical reaction over water. This makes it easy to...
Questions

Mathematics, 26.08.2020 22:01


Computers and Technology, 26.08.2020 22:01

Social Studies, 26.08.2020 22:01

Mathematics, 26.08.2020 22:01





History, 26.08.2020 22:01

Physics, 26.08.2020 22:01

Biology, 26.08.2020 22:01

Mathematics, 26.08.2020 22:01

Business, 26.08.2020 22:01

Mathematics, 26.08.2020 22:01


Biology, 26.08.2020 22:01


Mathematics, 26.08.2020 22:01