
At a certain temperature this reaction follows first-order kinetics with a rate constant of 0.0197s. 2N2O5 (g) right arrow 2N2O4 (g) + O2 (g)Suppose a vessel contains N2O5 at a concentration of 0.280 M. Calculate how long it takes for the concentration of N2Os to decrease by 0.0476 M. You may assume no other reaction is important.

Answers: 3
Another question on Chemistry

Chemistry, 23.06.2019 03:00
What happens in the particles of a gas when the gas is compressed
Answers: 1

Chemistry, 23.06.2019 03:40
Write the overall equation for the reaction occurring in lithium battery?
Answers: 3

Chemistry, 23.06.2019 06:00
Jenny wants to test the electrical conductivity of two substances dissolved in water. she is preparing the containers for the experiment. which factor is most important for her to control?
Answers: 1

Chemistry, 23.06.2019 08:00
How does the digestive system interact with the circulatory system? a. messages sent as electrical impulses from the digestive system are transported throughout the body by the circulatory system. b. nutrients taken in and broken down by the digestive system are carried to various parts of the body by the circulatory system. c. nutrients and gases are absorbed by organs in the circulatory system. then, they are transported to all parts of the body by organs in the digestive system. d. oxygen and carbon dioxide are exchanged by organs in the digestive system, and the gases are carried to the rest of the body by the circulatory system.
Answers: 2
You know the right answer?
At a certain temperature this reaction follows first-order kinetics with a rate constant of 0.0197s....
Questions














Chemistry, 28.11.2019 01:31






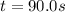
![ln(\frac{[N_2O_5]}{[N_2O_5]_0} )=-kt](/tpl/images/0693/5032/59694.png)
![t=\frac{ln(\frac{[N_2O_5]}{[N_2O_5]_0} )}{-k}=\frac{ln(\frac{0.0476M}{0.280M})}{-0.0197s}\\ \\t=90.0s](/tpl/images/0693/5032/59a2f.png)


