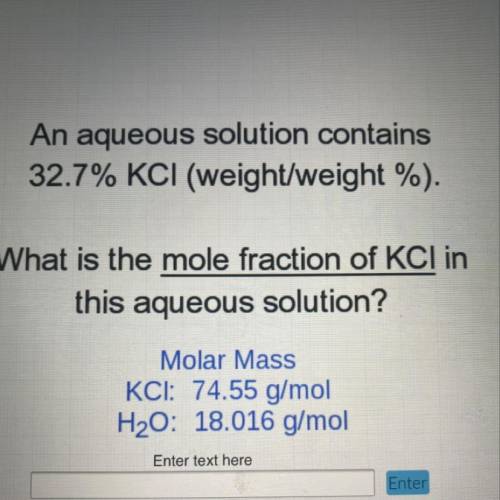
An aqueous solution contains
32.7% KCI (weight/weight %).
What is the mole fraction of KCI in...

Chemistry, 25.06.2020 02:01 angelequej1167
An aqueous solution contains
32.7% KCI (weight/weight %).
What is the mole fraction of KCI in
this aqueous solution?
Molar Mass
KCI: 74.55 g/mol
H2O: 18.016 g/mol
Enter text here
Enter

Answers: 1
Another question on Chemistry



Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1
You know the right answer?
Questions

Mathematics, 04.03.2020 02:12



Mathematics, 04.03.2020 02:12







Mathematics, 04.03.2020 02:13

Advanced Placement (AP), 04.03.2020 02:13

Mathematics, 04.03.2020 02:13


English, 04.03.2020 02:13



Mathematics, 04.03.2020 02:13

Social Studies, 04.03.2020 02:14

History, 04.03.2020 02:14



