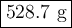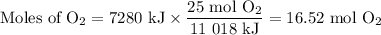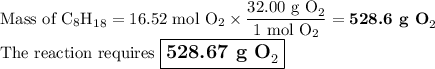Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:30
What problem would a person have if the nucleic acid in one of his or her cells were damaged?
Answers: 2

Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3

Chemistry, 22.06.2019 15:00
Why does a plastic bottle that is sealed at a high altitude change it’s shape when taken to lower altitude
Answers: 2

Chemistry, 22.06.2019 21:00
Which of these is an example of pseudoscience? a) predicting the time of sunrise based on data on position of earth b) predicting the date of the moon phases based on data on position of earth c) predicting eclipses based on the position of the sun and the moon d) predicting future events in a person's life based on the position of the moon
Answers: 1
You know the right answer?
A sample of octane undergoes combustion according to the equation 2 C8H18 + 25 O2 → 16 CO2 + 18 H2O...
Questions

Physics, 23.05.2021 20:30

Mathematics, 23.05.2021 20:30


Chemistry, 23.05.2021 20:30

Mathematics, 23.05.2021 20:30

Biology, 23.05.2021 20:30

French, 23.05.2021 20:30

History, 23.05.2021 20:30




Mathematics, 23.05.2021 20:40



Mathematics, 23.05.2021 20:40

Mathematics, 23.05.2021 20:40


Mathematics, 23.05.2021 20:40

Mathematics, 23.05.2021 20:40

Mathematics, 23.05.2021 20:40